Surgical hernia mesh
|
S-Pore |
M-Pore |
L-Pore |
|
S-Pore |
M-Pore |
L-Pore |
|
about 34 g/m2 |
about 35 g/m2 |
about 24 g/m2 |
PRODUCT CHARACTERISTICS
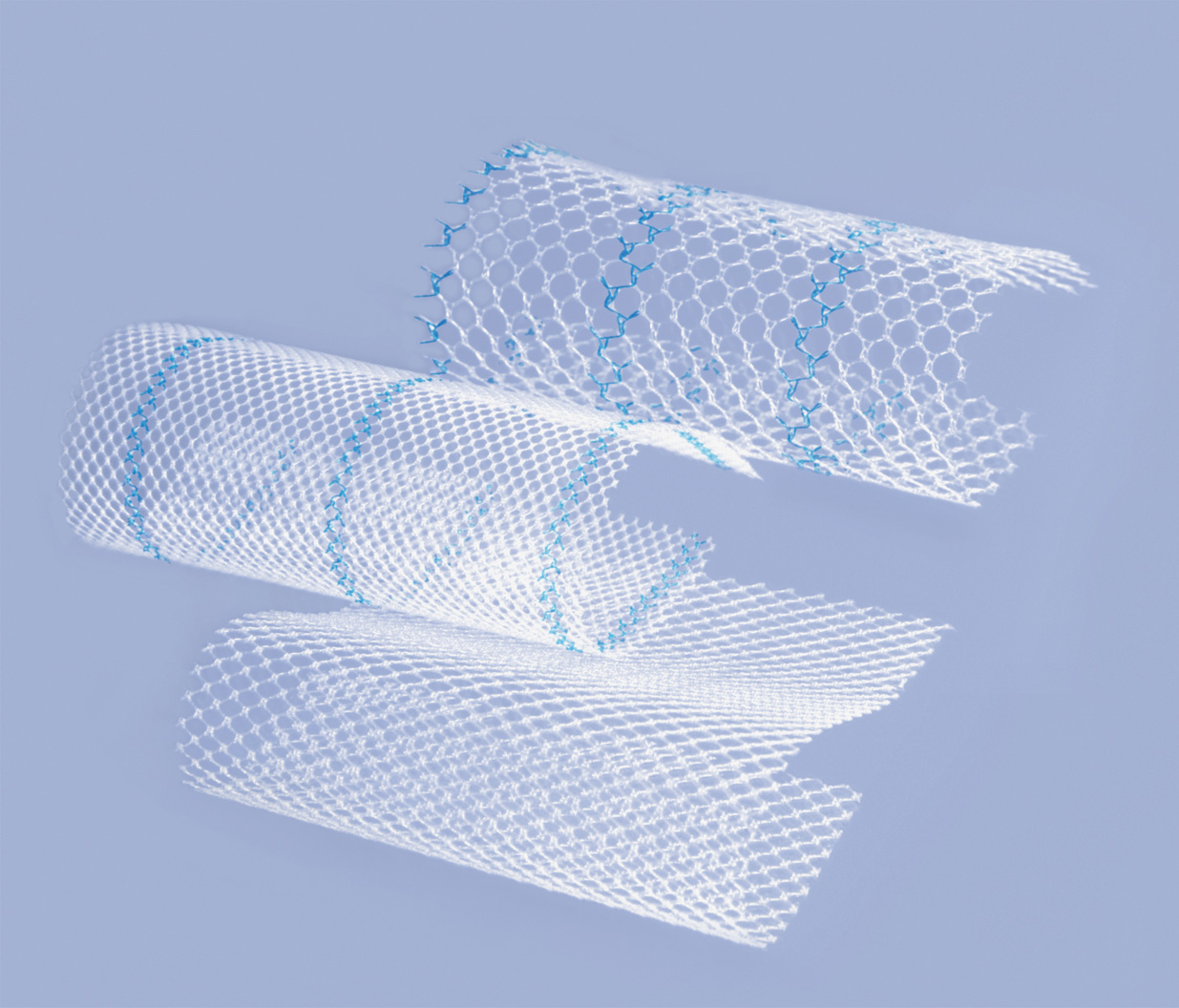
Optomesh® ULTRALIGHT non-resorbable surgical mesh products are knitted of transparent and blue monofilament polypropylene yarn using the knitting technique. They are designed for use in abdominal hernias.
Optomesh® ULTRALIGHT products are offered in three variants:
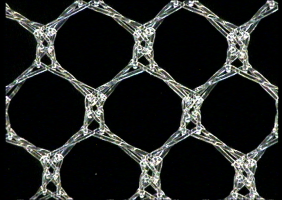
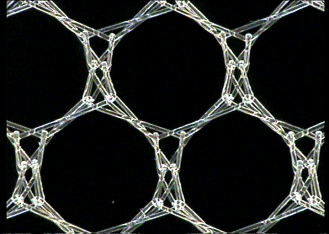
- L-Pore – mesh with very large pores (surface above 6 mm2);
- M-Pore – mesh with large pores (surface above 4 mm2);
- S-Pore – mesh with small pores (surface above 1.3 mm2).
Optomesh® ULTRALIGHT mesh implants are available in a full range of sizes, of various basis weight and mechanical properties which are adjusted to the size of the hernia gate. The meshes do not contain substances of allogenic and zoonotic origin.
The L-Pore and S-Pore variants have blue orienting lines facilitating the visibility and to positioning of the product in the operating field.
INDICATIONS
Optomesh® ULTRALIGHT surgical meshes are recommended for reconstructive procedures in order to strengthen places with soft tissue defect in the case of abdominal hernias i.e.:
- primary and recurrent,
- incisional hernias,
- inguinal and femoral hernias,
- umbilical hernias,
- with large gates.
Depending on the type and size of hernia the right type of surgical mesh can be chosen.
- Optomesh® ULTRALIGHT L-Pore type is recommended to be used for hernias with a small loss of connective (fascial) tissue [gate diameter up to 5 cm];
- Optomesh® ULTRALIGHT M-Pore type can be used for small and medium hernias [gate diameter up to 10 cm] as well as for large hernias [gate diameter above 10 cm];
- Optomesh® ULTRALIGHT S-Pore type can be used for small and medium hernias [gate diameter up to 10 cm].
Optomesh® ULTRALIGHT can be used for tension-free open surgical techniques (implant placed in an intraperitoneal position – onlay) and for laparoscopic techniques (implant placed in the preperitoneal position, behind the muscle-fascia layer – sublay).
CONTRAINDICATIONS
Optomesh® ULTRALIGHT should not be used in infected wounds or susceptible to infection and in conditions where it is not possible to maintain strict surgical asepsis. The Optomesh® ULTRALIGHT product is also not recommended in the case of the patient’s allergy/sensitivity to the product material (polypropylene).
Optomesh® ULTRALIGHT should not be used in children, women planning pregnancy, pregnant women, and where the process of physiological growth restricts the use of mesh products. It is not recommended to implant Optomesh® ULTRALIGHT in direct contact with internal organs due to the possibility of causing adhesions, fistulas or intestinal obstruction. Do not use the product if there is doubt about its sterility (e.g. soaking, cracked / damaged paper-film packaging, product discolouration).
PRECAUTIONS
- Optomesh® ULTRALIGHT is designed for use by qualified and trained medical personnel.
- In order to ensure strict surgical asepsis during the procedure, it is advisable to use special precautions (use of personal protective equipment, sterilisation of instruments, keeping high personal hygiene) and extraordinary attention while preparing the direct intervention site of the surgeon.
- Optomesh® ULTRALIGHT should only be used in non-infected wounds, and in conditions where it is possible to maintain strict surgical asepsis.
- Avoid direct contact of the mesh with internal organs.
- Do not implant the Optomesh® ULTRALIGHT surgical meshes after the expiration date.
- Optomesh® ULTRALIGHT polypropylene surgical meshes are offered for sale only in a sterile version. The product cannot be re-sterilised.
- Product for single use only.
- Do not use the product if it is damaged, with structural defects or contaminated.
FIXATION
Adjust the orientation of the L-Pore and S-Pore type to the mechanical properties of tissues. Lay the implant along the muscles in the direction with greater elasticity. It is recommended to lay the L-Pore and S-Pore variants in the transverse direction (blue orienting lines laid horizontally). Blue orienting lines facilitate the visibility of the product in the operating field, preventing fold formation and maintaining the implantation direction. The M-Pore type is characterised by similar properties of deformation in both directions.
For fixing the Optomesh® ULTRALIGHT mesh the use of surgical sutures, tackers (surgical screw-like tacks), tissue adhesive or staplers (surgical staples) is recommended. Absorbable fixing materials are also allowed. The size of the mesh and distance between the fixing places should be selected according to surgeon preferences, the nature of reconstruction and medical standards. It is allowed to cut the implant during a surgery to fit into the cavity. Using the fasteners the implant should be placed to closely adhere to the adjacent tissue. In order to prevent the recurrence of hernia, it is recommended to place the fixing elements at least approx. 1 cm from the edge of the product.
POSSIBLE COMPLICATIONS
The product application may cause the following complications: exudation of blood and serous fluid, infections, pain, discomfort in the wound, hematomas, bruises, recurrence of hernia, intestinal growth (formation of fistulas, adhesions, obstruction).
Diagnosed wound infections should be treated in the fastest possible way using available pharmacological methods. In case of severe and chronic infection, the possibility of partial resection of the infected tissue and/or the mesh should be considered. If there is no progress in the treatment of the infection, complete removal of the implant should be considered. The use of surgical tackers and staplers can cause chronic pain resulting from nerve compression. These complications can lead to a prolonged treatment process, chronic pain, recurrences, repeated surgical interventions or, in extreme cases, implant removal.
The use of a product that is non sterile, damaged or re-used can lead to the above-mentioned complications or serious health damage and patient’s life threat.
STERILISATION PROCESS
Sterilisation is carried out by means of ethylene oxide (EO) in a validated process.
The product maintains its sterility within a specified validity period, kept only in the original packaging at maintaining appropriate storage conditions.
STORAGE CONDITIONS
Optomesh® ULTRALIGHT should be stored:
- in dark rooms at humidity 25 – 65%,
- at temperature range 15-35oC,
- under conditions eliminating wetting and mechanical damage or chemical contamination,
- in the original unit packaging (box and direct packaging). Any mechanical damages to the packaging can expose the product to the loss of sterility.